Table of Content
Lidia’s experience includes serving as a senior research and policy analyst at the Association of American Medical Colleges on the Policy, Strategy & Outreach team. Lidia also practiced as a nurse at Georgetown University Hospital in the general medicine with telemetry unit and the GI endoscopy suite, where she assisted with endoscopic procedures and administered conscious sedation. Data provided via the Patient Access API would include all data classes and elements currently included in USCDI v.1. Payers would be required to report metrics about the use of Patient Access API. Security risk remains the only reason to deny an individual’s access request via Patient Access API.

Lidia Niecko-Najjumis a counsel in Crowell & Moring’s Health Care Group and is part of the firm’s Digital Health Practice. With over 15 years of clinical, policy, and legal experience, Lidia provides strategic advice on health care regulatory and policy matters, with particular focus on artificial intelligence, machine learning, digital therapeutics, telehealth, interoperability, and privacy and security. Representative clients include health plans, health systems, academic medical centers, digital health companies, and long-term care facilities.
Daylight Saving Time Info
The report does not include legislative recommendations, as additional analyses would need to be done prior to testing or universal implementation of a unified PAC payment system. The CMS 2023 final rule is better news for home health agencies than initially expected, but major concerns exist about whether the CMS payment adjustment methodology is consistent with the Medicare law on budget neutrality and will allow proper access to care over the long term. CMS proposes to require impacted payers to share certain prior authorization information through the Health Level 7® (HL7®) Fast Healthcare Interoperability Resources® (FHIR®) standard Patient Access API. Leslie Levinson is co-chair of the firm's Transactional Health Law Group and a member of both the Health Law and Business Transaction Groups.
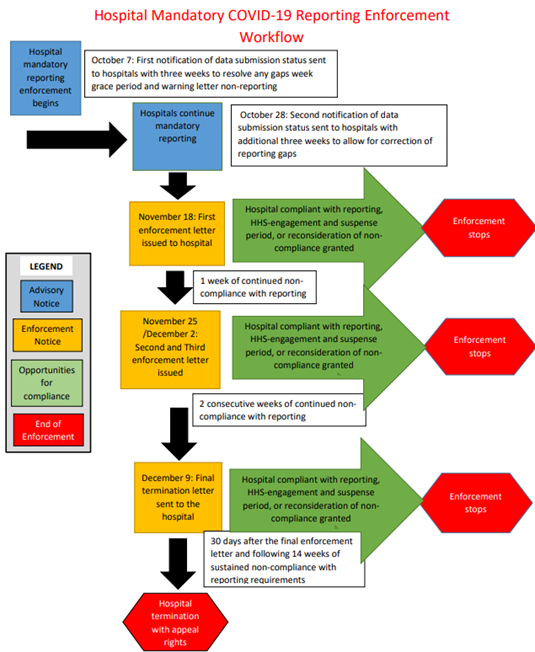
CMS reiterates in the Proposed Rule that the only reason payers could deny API access to a health app that a patient wishes to use and access through the Patient Access API is potential security risk to the payer. CMS enumerates that these security risks include insufficient authentication or authorization controls, poor encryption, or reverse engineering. The payer must make that determination using objective, verifiable criteria that are applied fairly and consistently across all apps and developers through which patients seek to access their electronic health information. The below summary does not focus on the Medicaid and Children’s Health Insurance Program Fee for Service proposals.
Home Health Prospective Payment System (HH PPS)
An impacted payer is required to respond to a non-impacted payer, however, if that non-impacted payer requests data exchange in accordance with the Proposed Rule. The Proposed Rule would rescind the payer-to-payer data exchange policy that did not impose a standard for the exchange, and proposes to require impacted payers to implement and maintain a payer-to-payer FHIR API to build a longitudinal patient record when the patient moves from one payer to another, or when the patient has concurrent coverage. While non-impacted payers may benefit from implementing the payer-to-payer API, they would not be under any obligation to do so. Therefore, the impacted payers in this Proposed Rule would only be responsible for their own side of the data sharing requests and responses. CMS is modifying current regulations to allow therapist assistants to perform maintenance therapy under the Medicare home health benefit in accordance with individual state practice requirements. This change is in response to comments received on a Request for Information in the CY 2018 HH PPS proposed rule on regulatory flexibilities and efficiencies.

HHAs must submit the zero-pay RAP within 5 calendar days of each 30-day period or be subject to a late penalty. For CY 2021, CMS is reducing administrative burden by streamlining the information required in order to submit the RAP. CMS believes that phasing out the RAP payments will mitigate potential fraud and is an important step in paying responsibly and appropriately for home health services.
Recent Updates
"These finalized measures are designed to improve patient safety by ensuring that the patient's medication list is provided to a provider and the patient as part of the discharge process," CMS said in a news release. Therapist assistants, as well as therapists, will be allowed to provide maintenance therapy under the Medicare home health benefit. In implementing the new model, CMS will use 30-day care periods rather than 60-day episodes of care as the payment unit. In response to recent occurrences of vaping related disorders, the NCHS is implementing a new diagnosis code, U07.0, Vaping-related disorder, into the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), for reporting vaping-related disorders. Attend this webinar to learn more about the new FINAL rule and how it will directly affect your Medicare home health agency. Agencies will do a better job in diagnostic coding, especially when there are two or more codes that equally meet the definition for the primary diagnosis.

Despite the reversal by CMS of its initially proposed steep payment cuts, many home health agencies and advocacy groups have expressed concern about the methodology used to arrive at the rate adjustments in the 2023 final rule. CMS had previously proposed a cut of 4.2 percent to 2023 payments, which would have been one of the steepest Medicare cuts in years. The previously proposed cuts were the first part of a total 7.69 percent payment cut to Medicare home health services beginning in 2023, as well as clawback cuts totaling $2 billion beginning in 2024 for services already provided during the first two years of the pandemic. One of the goals of the HHVBP Model is to enhance the current public reporting process for home health. CMS believes that publicly reporting HHVBP Model performance data would contribute to more meaningful and objective comparisons among HHAs on their level of quality relative to their peers, incentivize HHAs to improve their quality performance and could enable beneficiaries to make better informed decisions about where to receive care.
Time Zones - Frankfurt
The average and median time that elapsed between the submission of a request and a determination by the payer, plan, or issuer, for standard prior authorizations, aggregated for all items and services. The percentage of expedited prior authorization requests that were denied, aggregated for all items and services. The percentage of expedited prior authorization requests that were approved, aggregated for all items and services. The HHVBP Model is a value-based care test model required for all Medicare-certified HHAs providing services in Arizona, Florida, Iowa, Maryland, Massachusetts, Nebraska, North Carolina, Tennessee, and Washington. The Model adjusts Medicare payment rates up or down based on each HHA’s Total Performance Score in a given performance year, which is comprised of performance of certain measures and quality metrics. The HHA Rule requires publication of these scores on CMS’s website to allow consumers to evaluate competing HHAs, in an attempt to encourage quality care.

The average and median time that elapsed between the submission of a request and a decision by the payer, plan or issuer, for expedited prior authorizations, aggregated for all items and services. The foundational elements that make up PDGM remain unchanged from the proposed rule.Change to 30-day payment periods vs. 60-day. Certification episodes remain 60 days though with all OASIS timing points staying the same.
Finally, CMS is finalizing a requirement for a one-time submission of a Notice of Admission beginning in CY 2022 to replace RAP submissions every 30-days to further reduce provider paperwork burden while protecting the Medicare trust funds. HHAs must submit the NOA within 5 calendar days of the home health start of care or be subject to a payment penalty. On October 31, 2019, the Centers for Medicare and Medicaid Services finalizedits 2020 payment and policy changes rule for Home Health Agencies . The final rule is scheduled to be posted in the Federal Register on November 8, 2019, and allows for comments until December 30 in advance of its January 1, 2020, effective date. The HHA Rule makes changes to the Home Health Prospective Payment System , including the implementation of the Patient-Driven Groupings Model , and makes other policy changes for home health agencies to the Home Health Value-Based Purchasing Model and the Home Health Quality Reporting Program . These changes further the shift to a value-based payment system focusing on patient need over volume of care.

The NLR does not wish, nor does it intend, to solicit the business of anyone or to refer anyone to an attorney or other professional. NLR does not answer legal questions nor will we refer you to an attorney or other professional if you request such information from us. Our mission is to improve the lives of patients and providers by creating the most impactful educational content on an innovative learning platform. CMS will also begin collecting information on the use of telecommunications technology on home health claims in order to analyze the characteristics of beneficiaries utilizing remote services. This could provide insight into the social determinants of health, which influence who benefits most from those services and what barriers exist for differing subsets of patients. CMS will collect voluntarily starting on January 1, 2023, and collecting becomes mandatory on July 1, 2023.
CMS will group home infusion drugs into three payment categories, each with a unit of single payment, paid at amounts in accordance with specified infusion codes and units for such codes under the Physician Fee Schedule . CMS will adjust these payment amounts by the Geographic Adjustment Factor - a weighted composite of the three geographic practice cost indices used for the PFS. CMS also finalized paying higher payment amounts for the first home infusion therapy visit to account for costs to initiate these services. In response to public feedback, CMS is soliciting comments in this final rule with comment period on ways to enhance coverage of eligible drugs under the home infusion benefit. A home infusion drug must be administered intravenously in the home through a pump that is an item of DME.
No comments:
Post a Comment